IGCSE Chemistry Notes Solids, liquids and gases
Free IGCSE Chemistry Notes Solids, liquids and gases pdf download 2024 to 2028 Exams
1.1-IGCSE-Chemistry-Notes-States-of-Matter-Effect-of-temperature-and-pressure-on-the-volume-of-a-gas.pdf
1.1-IGCSE-Chemistry-Notes-States-of-Matter-Solid-liquid-and-gases.pdf

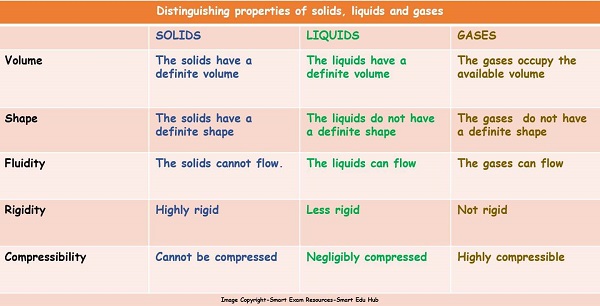
All matter is made up of mostly three types of particles; namely solids, liquids and gases.Following table summarises the distinguishing properties of solids, liquids and gases.
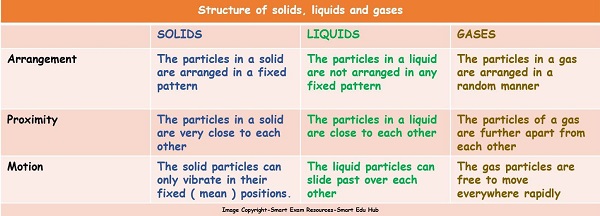
Following table summarises the structure of solids , liquids and gases:
Changes in state :
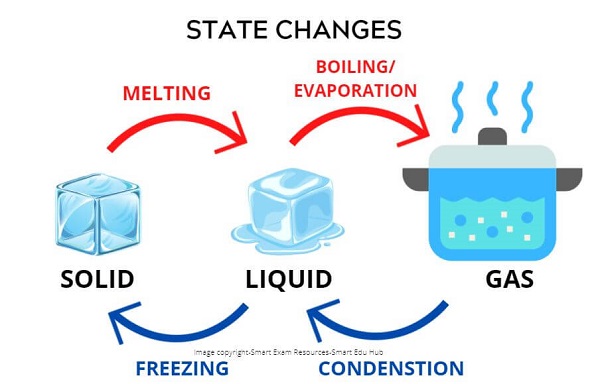
State Changes:
- Melting: It is a change from a solid to a liquid at the solid's melting point
- Boiling :It is a change of state from liquid to gas at the liquid's boiling point
- Evaporation: It is a change of state from liquid to gas at a range of temperatures
- Condensation: It is a change of state from gas to liquid at a range of temperatures
- Freezing: It is a change of state from liquid to gas at the liquid's freezing poin
THe effects of temperature and pressure on the volume of a gas
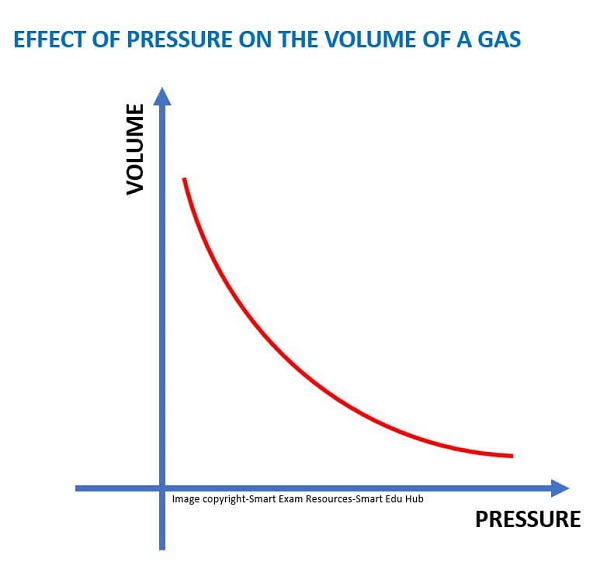
Describing the graph:
The volume of the gas decreases rapidly at first, as the pressure on it is increased. Then the volume almost reaches a constant.
Describing the volume changes of the gas:
The gas molecules are squashed together and hit the walls of the container . They come closer and the volume of the gas decreases.
Explain changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves
- Explain, in terms of kinetic particle theory, the effects of temperature and pressure on the volume of a gas.
Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves
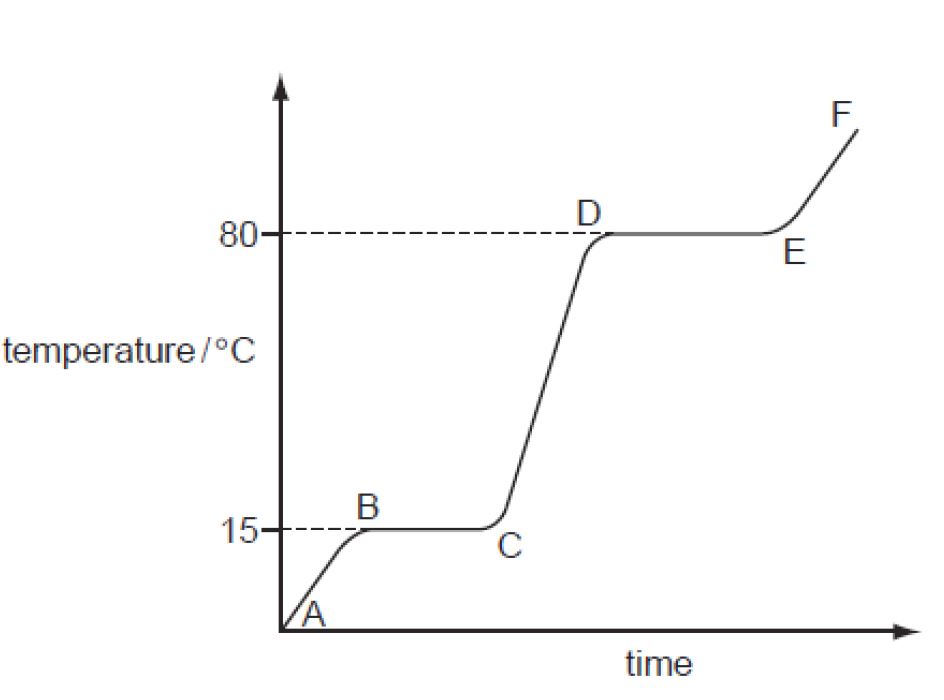
- Between A -B:The temperature of the solid increases. This is because increasing the heat energy increases the vibration of the particles in the solid.
- Between B-C: The force of attraction between the particles is weakened so the particles are able to slide past over each other. The temperature does not increase as all the heat supplied goes into overcoming the forces between the particles instead of raising the temperature. The substance melts.
- Between C-D. As time progresses the average kinetic energy of the liquid particles increases. Hence the temperature increases.
- Between D-E: The force of attraction between the particles is further weakened, so much so that the particles move well away from each other. The temperature is constant because the energy supplied goes into overcoming the forces between the particles instead of raising the temperature. The substance boils.
- Between E and F:The average kinetic energy of the particles increases and hence the speed of the particles also increases. Hence the temperature increases. The gas particles are now further away from each other.
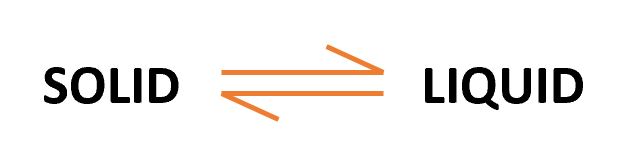
- Note: In the region BC, The equation of the equilibrium is as stated above
- The graph proves that a pure substance was used as the substance has a sharp melting point (at BC) and a sharp boiling point (at DE.)
- The temperatures 150C and 800C are important as they represent the melting and the boiling points.
- If an impure sample would have been used, the line BC would have been lower and the line DE would have been higher.
EFFECT OF TEMPERATURE AND PRESSURE ON THE VOLUME OF A GAS:
- According to the Kinetic theory, when the temperature increases, the average kinetic energy of the molecules also increases. and they begin to move faster.Keeping the volume of the gas constant, will lead to more frequent collisions of the gas molecules, with a greater force leading to an increase in the pressure
- Decreasing the temperature makes the gas particles move more slowly and the collisions become less hard and also less frequent. This leads to a decrease in pressure.
- Decreasing the volume of a gas increases the pressure of the gas. This is because , with a decrease in volume, the gas particles will have less space to move as the volume the gas occupied has been decreased.
- Increasing the volume of a gas decreases the pressure of the gas. This is because , with an increase in the volume, the gas particles will have more space to move as the volume the gas occupied has been increased.





